Warning! Science Ahead
This post attempts to bring some clarity to a few scientific concepts that are often overlooked or oversimplified in fire behavior training for firefighters and fire officers. I have made an effort to make this information accessible, but not to reduce it to the point where it no longer makes sense from a scientific perspective.
Fire Power
In physics, power is the rate at which work is performed or energy expended for a given unit of time. For combustion, power is the energy released per unit of time or heat release rate (HRR). So what? Why is this important to firefighters?
It is relatively easy to describe how big a compartment or building is based on its dimensions (i.e., length, width, height) in meters (or feet). However, describing how big a fire is requires different units of measure. Likely the best way to describe the size¯ of a fire is on the basis of the rate at which it is releasing energy.
In Heat Release Rate: A Brief Primer, Dr. Vytenis Babrauskas observes that Heat Release Rate (HRR) is the driving force that influences many other dimensions of the fire environment. As HRR increases, temperature and the rate of temperature change both increase, accelerating fire development. In addition, increased HRR results in reduced oxygen concentration and increased production of gaseous and particulate products of incomplete combustion. For firefighters, it is also important that HRR directly relates to flow rate required for fire control.
Measuring Energy and Power
Energy is often defined as the ability to do work or cause change. Thermodynamic work is the transfer of energy from one system to another. This is sometimes, but not always accompanied by an increase in temperature (more on this in a bit).
In the United States, the traditional units of measure for energy were the British thermal unit (Btu). A Btu is the amount of energy required to raise the temperature of one pound of water from 60o F to 61o F. Adding additional Btu will continue to raise the temperature of the water until it reaches its boiling point. Changing phase from liquid to gas requires input of a large amount of energy, but there is no change in temperature!
The standard international (SI) unit for energy is the Joule (J). The joule is defined in terms of mechanical energy. However, in our context, it is useful to describe the Joule in terms of transfer of thermal energy. 4186 J will raise the temperature of 1 kilogram (kg) of water from 20o C to 21o C. For readers who are more comfortable with Btu, one Btu is equal to 1055 J (slightly more than one kilojoule (kJ)).
Power is the rate at which work is performed or energy is transferred. This necessitates a measure of the amount of energy (i.e., Btu or J) and a unit of time (generally minutes or seconds). Using traditional units, power could be described in terms of Btu/minute or Btu/second. Watts are the SI unit for power, with a Watt being a Joule/second (J/s)
To keep things simple, the remainder of this post will stick to the SI units (Joules, Watts, and oC).
Potential Energy of Fuel
Energy that is stored is known as potential energy. Fuel has chemical potential energy that is released as the fuel is oxidized in the combustion process. The energy that is released through complete combustion of a given mass of fuel is known as the heat of combustion. Heat of combustion is dependent on the chemical makeup of the fuel. Heat of combustion is usually expressed in kilojoules/gram (kJ/g) or megajoules/kilogram (MJ/kg).
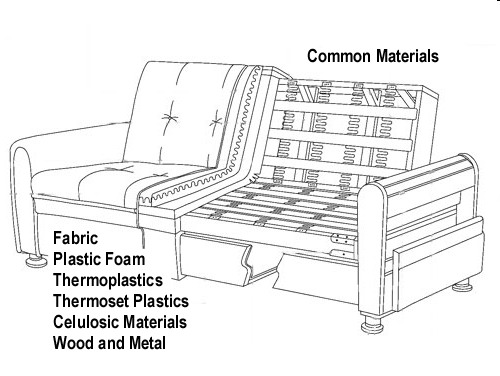
Generally (hydrocarbon based) synthetic fuels have a higher heat of combustion than cellulose fuels such as wood as illustrated in the following table:

Note: Data in this table is from the Society of Fire Protection Engineering (SFPE) Handbook of Fire Protection Engineering.
When fuel burns, the total energy that can be released is dependent on its heat of combustion and fuel mass (e.g., kg of fuel)
Heat of combustion is important, but as Dr. Babrauskas points out, the rate at which that energy is released is even more important. Heat release rate is influenced by a number of different fuel characteristics such as surface area to mass ratio, orientation (e.g., horizontal, vertical), arrangement, and geometry.
The concepts of heat of combustion and heat release rate help explain changes in the built environment that impact firefighting. Increased use of synthetic materials has increased the chemical potential energy of building materials and contents and higher heat release rates shorten time to flashover.
Oxygen and Combustion
Release of chemical potential energy from fuel depends on availability of adequate oxygen for the combustion reaction to occur. Interestingly, while the heat of combustion of various types of organic (carbon based) fuel varies widely, the amount of oxygen required for release of a given amount of energy remains remarkably consistent.
In 1917, British scientist W.M. Thornton discovered that the amount of oxygen required per unit of energy released from many common hydrocarbons and hydrocarbon derivatives is fairly constant. In the 1970’s, researchers at the National Bureau of Standards independently discovered the same thing and extended this work to include many other types of organic materials and examined both complete and incomplete combustion.
Each kilogram of oxygen used in the combustion of common organic materials results in release of 13.1 MJ of energy. This is referred to as Thornton’s Rule.
However, the concentration of oxygen in the atmosphere is only 21%. Examining the relationship between consumption of atmospheric oxygen and energy release requires adaptation of Thornton’s Rule based on oxygen concentration. Multiplying 13.1 MJ/kg of oxygen by 21% gives a value of 2.751 MJ/kg of air. The Society of Fire Protection Engineering (SFPE) Handbook of Fire Protection Engineering rounds this value to 3.0 MJ/kg of air. While it is easy to understand that air has mass, it is a bit more difficult to visualize a kilo of air! The density of dry air at sea level and at a temperature of 20o C is 1.2 kg/m3 (0.075 lbs./ft3). Air density decreases as temperature or moisture content of the air increases, but this provides a starting point for visualizing the relationship between volume and mass at normal temperature and pressure.
All this is very interesting, but how does it relate to compartment fires and firefighting?
Fuel and Ventilation
In a compartment fire, combustion occurs in an enclosure where the air available for combustion is limited by 1) the volume of the compartment and 2) ventilation.
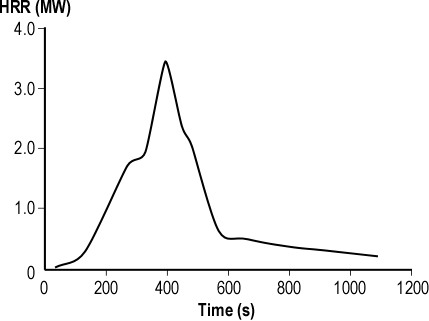
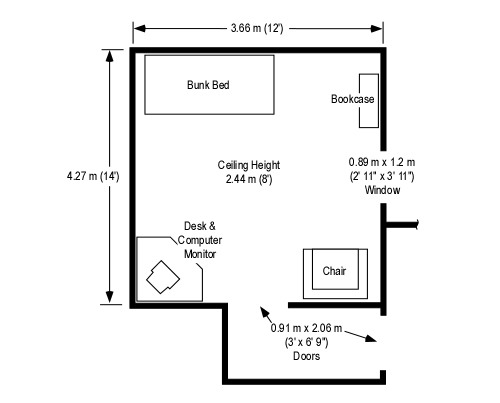
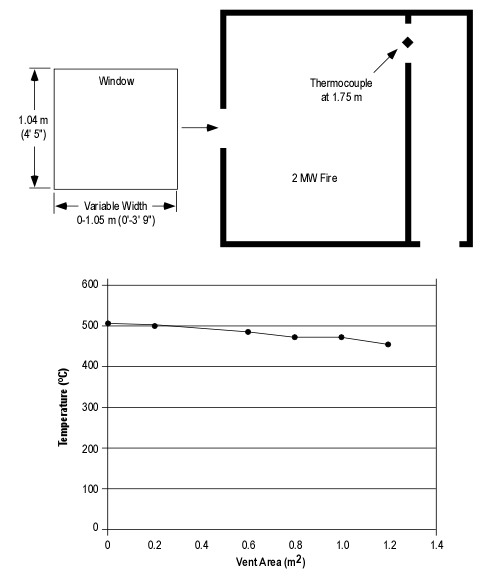
Consider a 2.4 m x 3.7 m (8′ x 12′) compartment with a ceiling height of 2.4 m (8′). A compartment of this size has a volume of 21.312 m3 (752.63 ft3). Based on a potential heat release of 3 MJ/m3 of air, the volume of the compartment would provide sufficient air for release of 63.936 MJ. A fire burning in this compartment with a steady heat release rate of .5 MW would consume the air in the compartment in just over two minutes (127.8 seconds). However, this is an extreme oversimplification as fires generally begin with a low heat release rate and grow until they become limited by the availability of fuel or oxygen. In this case, the fire would burn for a bit longer and would then cease flaming combustion, but surface combustion may (depending on the type of fuel involved) continue for some time after the oxygen concentration drops below 15%.
It is unlikely that a fire would occur in a compartment that had no openings (or at least potential openings) such as a door and one or more windows. Even if these openings are closed, there will likely be some leakage that will influence the amount of air available to support combustion. If they are open, a substantially greater amount of air will be available to support fire growth. However, as the fire develops and a hot gas layer forms and begins to fill the compartment, exiting smoke reduces the size of the opening serving as an inlet for additional air. As this occurs, the fire becomes ventilation controlled and heat release is limited by the amount of oxygen in the air available to support combustion.

Note: Photos adapted from National Institute of Standards and Technology (NIST) ISO-Room/Living Room Flashover.
Hazard of Ventilation Controlled Fires
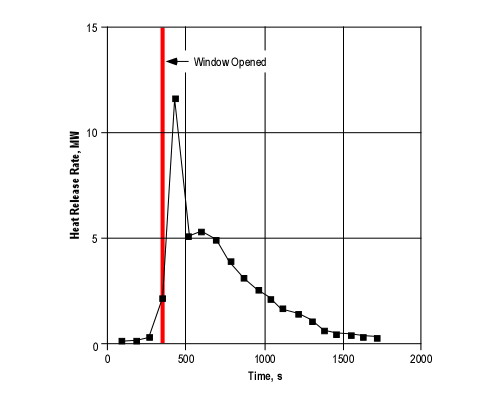
Many if not most fires that have progressed beyond the incipient stage when the fire department arrives are ventilation controlled. This means that the heat release rate (the fires power) is limited by the ventilation profile, in particular, the existing openings.
If ventilation is increased, either through tactical action or unplanned ventilation resulting from effects of the fire (e.g., failure of a window) or human action (e.g., exiting civilians leaving a door open), heat release rate will increase.
Ventilation is a complex strategy as it can have both positive and negative effects. Releasing smoke can make the interior environment more tenable by raising the level of the hot gas layer and removing energy and fuel (hot smoke) from the compartment or building. However, increasing the air supply to a ventilation controlled fire will increase the heat release rate, potentially resulting in a ventilation induced flashover.
It is essential that firefighters and fire officers understand the effects of tactical operations on fire behavior and coordinate their efforts to maximize the positive impact while limiting the negative consequences.
Chief Pete Lamb recently wrote a blog post titled Vent Early in which he emphasizes the need for firefighters to understand the application of ventilation strategies and to use them effectively. I suggest that we vent wisely in coordination with fire attack after considering fire behavior and building factors! Understanding compartment fire behavior and practical fire dynamics is critical to safe and effective ventilation operations.
If you found this post interesting or useful (or not), please leave a comment with your feedback.
Ed Hartin, MS, EFO, MIFireE, CFO