Things to Think About
Methods of heat transfer are often presented to firefighters in a simplistic way with the expectation that they will understand the basic concepts and are assessed on their ability to recall the definitions of conduction, convection, and radiation. Unfortunately this does not provide a solid basis for understanding phenomena encountered on the fireground.
Convection
In general terms, convection refers to movement of molecules within fluids (i.e., liquids and gases). Convection results in both heat and mass transfer (these are interrelated as extensive properties such as thermal energy are dependent on mass). Convection involves diffusion due to random movement of individual molecules (Brownian motion) and large scale motion of currents in the fluid (advection).
Natural Convection
Natural or free convection results from temperature differences within a fluid. As a fluid is heated, it expands while mass remains the same. Decreased density (mass/unit volume) makes the heated fluid more buoyant, causing it to rise. As the heated fluid rises, cooler fluid flows in to replace it. Natural convection is one of the major mechanisms of heat transfer in a compartment fire, heated air and smoke rise and cooler air moves in to replace it. This process transfers thermal energy, heating other materials in the compartment and also transfers mass as smoke moves out of the compartment and cool air (containing oxygen necessary for continued combustion) moves into the compartment.
In the late 1700s, French scientist Jacques Charles studied the effect of temperature on a sample of gas at a constant pressure. Charles, found that as the gas was heated, the volume increased. As the gas was cooled, the volume decreased. This finding gave rise to Charles Law.
Charles’ Law: At a constant pressure, the volume occupied by a fixed mass of gas is directly proportional to its thermodynamic temperature (V?T).
Figure 1. Charles Law
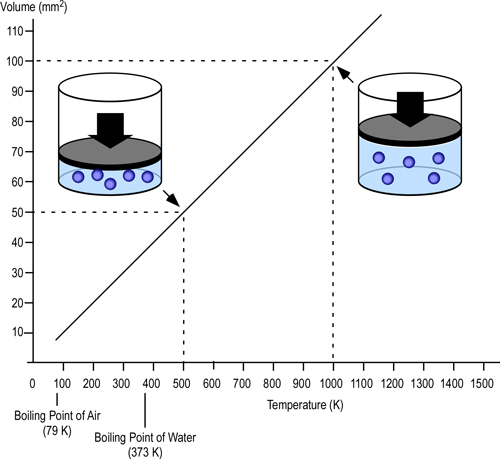
Density is mass per unit volume (?=m/V). As the volume of a given mass of gas increases, it becomes less dense (and more buoyant). If unconfined, gases that are less dense than the surrounding air will rise (resulting in natural convection currents). In a compartment fire, conditions are not as simple as stated in Charles Law. Initially, hot gases resulting from a fire in a compartment are relatively unconfined as the volume of smoke and hot gases is small in relation to the size of the compartment and there may be some leakage of smoke from the enclosure. However, as the fire continues to develop, the volume of smoke increases and conditions change.
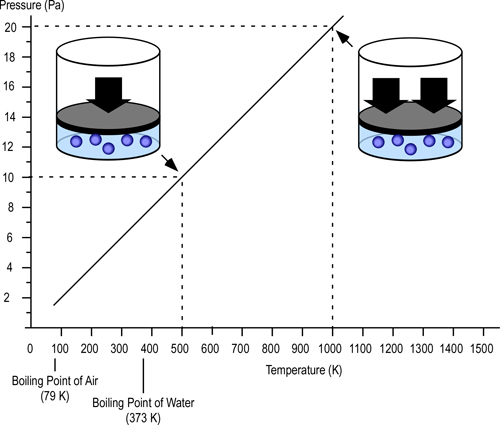
Gay-Lussac’s Law: At a constant volume and mass, the pressure exerted by a gas is directly proportional to its thermodynamic temperature (P?T).
The pascal (Pa) is the standard international unit of measure for pressure (force per unit area) and is defined as one newton per square meter (N/m2). To provide a point of reference for firefighters more familiar with pounds per square inch (psi) as a unit of measure for pressure, 1 Pa = 0.000145 psi. Low pressures (such as the pressure generated by temperature increases in a compartment fire) are measured in Pa while higher pressures (such as fire pump discharge pressure) are more commonly measured in kilopascals (kPa=1000 Pa).
As illustrated in Figure 2, if the volume is constant (e.g., the gas is confined) doubling the temperature in Kelvins, doubles the pressure in pascals (Pa).
Figure 2. Gay-Lussac’s Law
When a fire is unconfined (e.g., outdoors), convection is influenced primarily by differences in density between hot fire gases and cooler air. Convection as a result of a fire in an enclosure (e.g., compartment fire) is significantly influenced by differences in density and differences in pressure.
Figure 3. Natural Convection

Forced Convection
In forced convection, energy is carried passively by fluid motion which occurs independent of the heating process.
While at first glance, it may appear that this type of convection would not be encountered in the fire environment, but it is extremely important. Forced convection can be caused by natural effects such as wind blowing into an opening (e.g., window broken due to fire effects). This type of forced convection can quickly create untenable conditions both inside the compartment and in adjacent spaces (e.g., rooms, hallways). Forced convection can also have a positive influence on the fire environment. One example would be the use of positive pressure ventilation, in which a blower (fan) is used to create an air flow from an inlet to an exhaust opening, removing hot smoke and gases from the compartment.
Figure 4. Forced Convection/Wind Driven Fire

Note: Adapted from Fire Fighting Tactics Under Wind Driven Fire Conditions: 7-Story Building Experiments.
Factors Influencing Convective Heat Transfer
Heat transfer by convection is more complex than conduction as there is no single property such as thermal conductivity that can be used to describe the mechanism of heat transfer. Factors that influence heat transfer by convection in the fire environment include temperature difference between the fluid (gas) and surfaces, fluid velocity, and turbulence (related to surface roughness and compartment configuration).
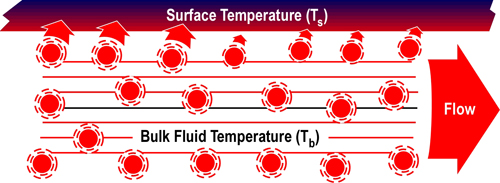
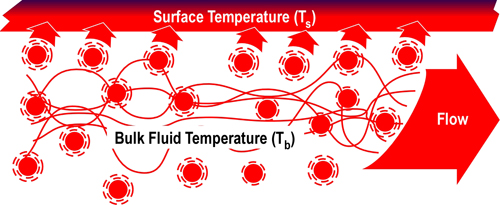
Figure 5 illustrates convective heat transfer with laminar (smooth) fluid flow. Energy is transferred from higher temperature fluid molecules to the cooler surface. Bulk fluid temperature (Tb) is the temperature of the fluid some distance away from the surface. As heat is transferred, the temperature of the fluid molecules is lowered (with a corresponding rise in surface temperature). These cooler molecules insulate the surface from the higher temperature molecules further away from the surface, slowing convective heat transfer.
Figure 5. Convection-Laminar Flow
When velocity and/or turbulence increases, cooler molecules that have transferred energy to the surface are quickly replaced by higher temperature molecules, resulting in increased convective heat transfer as illustrated in Figure 6. This is the same phenomena as wind chill, except in this case, energy is transferred from a hot fluid (gas) to a solid surface rather than from a hot surface (i.e., your skin) to a cooler fluid (air).
Figure 6. Convection-Turbulent Flow
More to Follow
Subsequent posts in this series will examine radiant heat transfer and then move on into discussion of the process of combustion.
Ed Hartin, MS, EFO, MIFireE, CFO
References
Kerber, S. & Madrzykowski, D. (2009) Fire Fighting Tactics Under Wind Driven Fire Conditions: 7-Story Building Experiments, NIST Technical Note 1629. Gaithersburg, MD: National Institute of Standards and Technology.