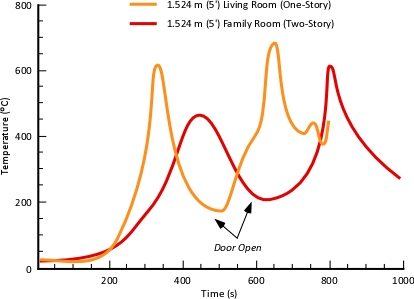
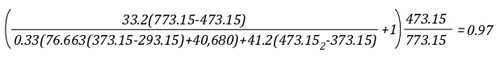“Flashover Training”
Saturday, April 6th, 2013This week’s questions focus on training firefighters to recognize, prevent, and if necessary react appropriately to flashover conditions. Casey Lindsay of the Garland, Texas Fire Department sent an e-mail to a number of fire behavior instructors regarding how they conduct “flashover training”
One of the challenges we face in discussing fire behavior training, particularly live fire training is the result of variations in terminology. Differences exist in the way that live fire training props are described and in fire control techniques. For this discussion, CFBT-US defines the type of prop pictured below as a “split level demo cell”. This terminology is derived from the original purpose of this design as conceived by the Swedish Fire Service in the 1980s. The split level cell is intended for initial fire behavior training focused on observation of fire development. As used in the United States (and some other parts of the world) it is described as a “flashover simulator” or “flashover chamber”. This provides a disconnect in context as this prop is not intended and does not subject the participants in training to flashover conditions, but simply provides an opportunity to observe fire development through the growth stage and recognize some potential cues of impending flashover.

Note: The prop illustrated above is a Split level cell at the Palm Beach County Fire Training Center.
Container based props can be configured in a variety of ways for both demonstration and fire attack training. Most commonly single compartment cells are single level or split level design. Multiple compartment cells are arranged in a variety of ways with containers placed in an “L”, “H” or other configuration.
Do you currently teach firefighters that “Penciling control techniques can be used to give firefighters additional time to escape a flashover”?
We define penciling as an intermittent application using a straight stream as compared to pulsing which uses a fog pattern or painting which is a gentle application of water to hot surfaces. We do not teach penciling, pulsing, or painting as a technique to give firefighters additional time to escape flashover. We use gas cooling (short or long pulses) and coordination of fire attack and ventilation to control the environment and prevent or reduce the potential for firefighters to encounter flashover. However, long pulses (or continuous application) while withdrawing is taught as a method of self-protection if fire conditions exceed the capability of the crew engaged in fire attack.
In response to Casey’s questions, Jim Hester, with the United States Air Force (USAF) presents an alternative perspective:
No! We do not teach penciling or 3D Fog attack anymore. We did temporarily after receiving our training as instructors in the flashover trainer. We gave the technique an honest look and conducted research using Paul Grimwood’s theories. We decided there are too many variables. For example; what works in a room and contents [fire] will not work in heavy fire conditions inside a commercial. The last thing we want is someone penciling any fire, inside any structure, that requires constant water application until the fire is darkened down. That’s what we teach. Open the nozzle for as long as it takes to get knock down and then shut the nozzle down. [It is as] simple as that. If you take that approach, even in the flashover trainer you will alleviate confusion or misapplication of your fire stream.
While I have a considerably different perspective, Jim raises several good points. I agree that there are many variables related to fire conditions and room geometry. If firefighters are trained in lock step manner that short pulses are used to control the temperature overhead, there will definitely be a challenge in transitioning from the container to a residential fire and even more so when confronted with a commercial fire. However, if firefighters are introduced to the container as a laboratory where small fires are used to develop understanding of nozzle technique, rather than a reflection of real world conditions, this presents less of an issue.
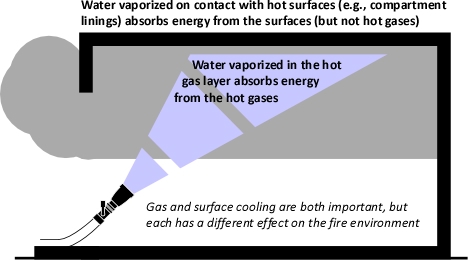
As Jim describes, fire conditions requiring constant application in a combination attack with coordinated tactical ventilation, may not be controlled by short pulses. However, when cooling hot smoke on approach to a shielded fire, constant application of water will likely result in over application and less tenable conditions (too much water may not be as bad as too little, but it presents its own problems).
Most firefighters, even those that advocate continuous application, recognize that a small fire in a trash can or smoldering fire in a upholstered chair or bed does not require a high flow rate and can easily be controlled and extinguished with a small amount of water. On the other hand, a fully developed fire in a large commercial compartment cannot be controlled by a low flow handline. To some extent this defines the continuum of offensive fire attack, small fires easily controlled by direct application of a small amount of water and large fires that are difficult to control without high flow handlines (or multiple smaller handlines). There is not a single answer to what is the best application for offensive fire attack. Shielded fires require control of the environment (e.g., cooling of the hot upper layer) to permit approach and application of direct or combination attack. Fires that are not shielded present a simpler challenge as water can be brought to bear on the seat of the fire with less difficulty.
Nozzle operators must be trained to read conditions and select nozzle technique (pulsed application to cool hot gases versus penciling or painting to cool hot surfaces) and fire control methods (gas cooling, direct attack, indirect attack, or combination attack) based on an assessment of both the building and fire conditions.
What flashover warning signs do you cover during the classroom portion of flashover training?
We frame this discussion in terms of the B-SAHF (Building, Smoke, Air Track, Heat, and Flame) indicators used in reading the fire (generally, not just in relation to flashover).

Building: Flashover can occur in all types of buildings. Consider compartmentation, fuel type, and configuration, ventilation profile, and thermal properties of the structure. Anticipate potential for increased ventilation (without coordinated fire control) to result in flashover when the fire is burning in a ventilation controlled regime (most fires beyond the incipient stage are ventilation controlled). Note that these indicators are not all read during the incident, but are considered as part of knowing the buildings in your response area and assessing the building as part of size-up.
Smoke: Increasing volume, darkening color and thickness (optical density), lowing of the level of the hot gas layer.
Air Track: Strong bi-directional (in at the bottom and out at the top of an opening), turbulent smoke discharge at openings, pulsing air track (may be an indicator of ventilation induced flashover or backdraft), and any air track that shows air movement with increasing velocity and turbulence.
Heat: Pronounced heat signature from the exterior (thermal imager), darkened windows, hot surfaces, hot interior temperatures, observation of pyrolysis, and feeling a rapid increase in temperature while working inside (note that this may not provide sufficient warning in and of itself as it is a late indicator).
Flame: Ignition of gases escaping from the fire compartment, flames at the ceiling level of the compartment, isolated flames in the upper layer (strong indicator of a ventilation controlled fire) and rollover (a late indicator).
How do you incorporate the thermal imaging camera into your flashover class?
We do not teach a “flashover” class. We incorporate learning about flashover (a single fire behavior phenomena) in the context of comprehensive training in practical fire dynamics, fire control, and ventilation (inclusive of tactical ventilation and tactical anti-ventilation). Thermal imagers (TI) are used in a variety of ways beginning with observation of small scale models (live fire), observation of fire development (with and without the TI) and observation of the effects of fire control and ventilation.
Do you allow students to operate the nozzle in the flashover chamber?
We use a sequence of evolutions and in the first, the students are simply observers watching fire development and to a lesser extent the effects of water application by the instructor. In this evolution, the instructor limits nozzle use and predominantly sets conditions by controlling ventilation. If necessary the instructor will cool the upper layer to prevent flames from extending over the heads of the participants or to reduce the burning rate of the fuel to extend the evolution. Students practice nozzle technique (short and long pulses, painting, and penciling) outside in a non-fire environment prior to application in a live fire context. After the initial demonstration burn, students develop proficiency by practicing their nozzle technique in a live fire context.
When working in a single level cell rather than a split level cell (commonly, but inaccurately referred to as a “flashover chamber” or “flashover simulator”) we expand on development of students proficiency in nozzle technique by having them practice cooling the upper layer while advancing and importantly, while retreating. In addition, students practice door entry procedures that integrate a tactical size-up, door control, and cooling hot gases at the entry point.
Do you maintain two-in/two-out during flashover chamber classes?
We comply with the provisions of NFPA 1403 and provide for two-in/two-out by staffing a Rapid Intervention Crew/Company during all live fire training.
What is your fuel of choice for the 4×8 sheets (OSB, Particleboard or Masonite)?
We have used a variety of fuel types, but commonly use particle board. OSB tends to burn quickly, but can be used if this characteristic is recognized. We have also used a low density fiberboard product (with less glue) which performs reasonably well. The key with fuel is understanding its characteristics and using the minimum quantity of fuel that will provide sufficient context for the training to be conducted. I recommend that instructors conduct test burns (without students) when evaluating fuel packages that will be used in a specific burn building or purpose built prop (such as a demo or attack cell).
Do you have benches or seating in the flashover chamber?
No, firefighters are expected to be in the same position that they would on the fireground, kneeling or in a tripod position. When we work in a demo cell (“flashover chamber”) with benches, we keep the students on the floor.
Do you teach any flashover survival techniques, other than retreat/evacuate?
We focus first on staying out of trouble by controlling the environment. Second, we teach firefighters the skill of retreating while operating the hoseline (generally long pulses to control flames overhead). There are not really any options other than control the fire of leave the environment (quickly)! This is similar to James Hester’s answer of continuous flow, with a sweeping motion (long pulses can be applied in a sweeping manner, particularly in a large compartment). It is important to understand that a short pulse is extremely short (as fast as you can open the nozzle) and a long pulse is anything else (from several seconds to near continuous application, depending on conditions).
Refer to the series of CFBT Blog on Battle Drills for additional discussion developing proficiency in reaction to deteriorating conditions.
Additional Thoughts
Our perspective is that discussion of flashover should be framed in the context of comprehensive fire behavior training, rather than as a “special” topic. Practical fire dynamics must be integrated into all types of structural firefighting training, in particular: Hose Handling, Fire Control, and Tactical Ventilation (but the list goes on). When working with charged hoselines, take the time to practice good nozzle technique as well as moving forward and backward (do not simply stand up and flow water when performing hose evolutions). In fire control training (live fire or not), practice door control, tactical size-up, and door entry procedures. When training on the task activity of tactical ventilation (e.g., taking glass or cutting roof openings), make the decision process explicit and consider the critical elements of coordination and anticipated outcome of you actions.
FDIC
Plan on attending Wind Driven Fires in Private Dwellings at Fire Department Instructors Conference, Indianapolis, IN on Wednesday April 24, 2013 in Wabash 3. Representing Central Whidbey Island Fire & Rescue, Chief Ed Hartin will examine the application of NIST research on wind driven fires to fires in private dwellings. This workshop is a must if the wind blows where you fight fires!