Everyday Concepts-Part 4
Radiation
Monday, April 12th, 2010
Firefighters are often provided with an oversimplified explanation of fundamental scientific concepts related to fire behavior. This is done with the intent to make training manuals and texts understandable and to focus on the information that firefighters “must know”. However, a tremendous opportunity to develop the ability to make sense of fire dynamics and the impact of tactical operations is lost in the process. This series of posts continues to explore ways of building a scaffold to allow firefighters to develop a deeper understanding of firefighting as science.
Electromagnetic Radiation
The term radiation is used to describe many different things ranging from visible light, infrared light, and ionizing radiation such as x or gamma rays. Each of these is an example of radiation as an electromagnetic wave produced by the motion of electrically charged particles. Electromagnetic radiation can travel through empty space and air. Radiation can also penetrate through other materials depending on the characteristics of the material and the radiation’s energy. Some ionizing radiation is in the form of particles (rather than waves), but that is outside the scope of our examination of radiation as a mechanism of heat transfer.
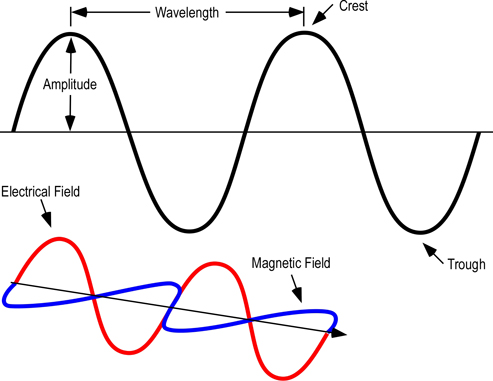
As illustrated in Figure 1, electromagnetic waves can be described in terms of their wavelength, amplitude, frequency, and energy.
Most of the electromagnetic spectrum cannot be detected by the human eye. While the electromagnetic spectrum includes radiation in a broad range of wavelengths, those of most interest in the study of fire behavior are categorized as infrared
Figure 1. Electromagnetic Wave
From longest to shortest wavelengths, the spectrum is usually divided into the following sections: radio, microwave, infrared, visible, ultraviolet, x-ray, and gamma-ray radiation. Humans can only see a narrow band of visible light, which is a small fraction of the electromagnetic spectrum. We perceive this radiation as the colors of the rainbow ranging from red to violet, with reds having longer wavelengths and violet having shorter wavelengths
Thermal radiation is electromagnetic radiation emitted from the surface of an object which is due to the object’s temperature. Any material that is above absolute zero gives off some radiant energy. Thermal radiation is generated when heat from the movement of charged particles within atoms is converted to electromagnetic radiation.
Figure 2. Electromagnetic Spectrum
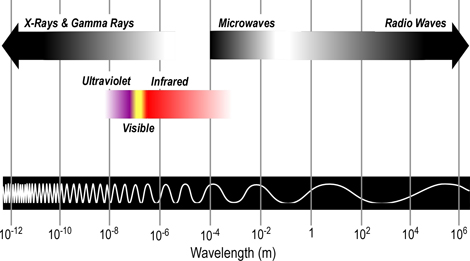
Figure 3. Planck’s Curve
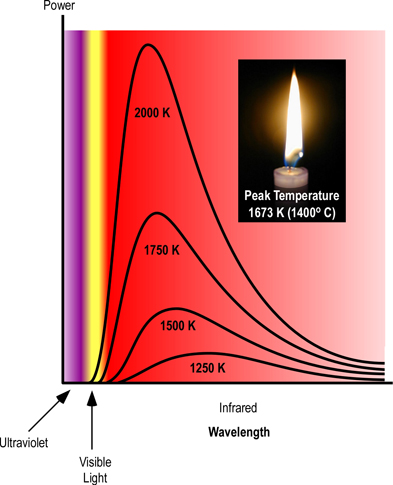
Thermal radiation occurs at a wide range of frequencies. However, as illustrated in Figure 2, the power emitted at each wavelength is dependent on temperature, with the main frequency and power of emitted radiation increasing as temperature increases. This can be observed when color changes from red, to yellow, and then white as an object is heated. While color change is visible, most of the radiant energy is still in the infrared spectrum.
Electromagnetic waves of any frequency will heat surfaces that absorb them. However, temperatures of hot surfaces, gases, and flames in the fire environment result in emission of electromagnetic waves predominantly in the infrared and visible portion of the spectrum.
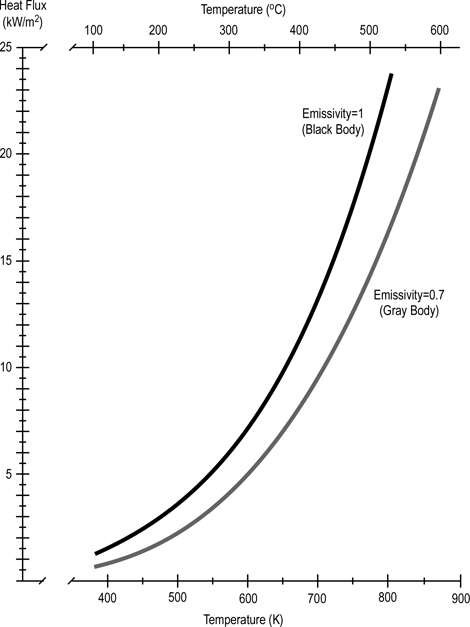
Stefan-Boltzmann Law: The amount of energy per square meter per second that is emitted by a black body is related to the fourth power of its Kelvin temperature. As temperature increases, emission of radiant energy increases exponentially.
A black body is a theoretical object that completely absorbs all incoming radiant energy and is also a perfect emitter of radiant energy. Materials encountered in the fire environment do not completely have the characteristics of a black body and may be classified as gray bodies. A gray body absorbs or emits a portion of the radiative flux depending on the emissivity (?).
Emissivity is the relative ability of the surface of a material to emit radiant energy. It is the ratio of energy radiated by a particular material to energy radiated by a black body at the same temperature. Emmisivity of a black body would be 1.0 with the emissivity of actual materials ranging from approximately 0.1 for highly reflective materials (e.g., polished silver) to 0.97 for fairly efficient absorbers and emitters of radiant energy (e.g., carbon particulate).
Emission of radiant energy is measured as heat flux (energy transfer per unit of time over a given surface area). The SI unit of measure is joules/second/square meter or (since a watt is a J/s) watts/square meter (w/m2).
Figure 4. Stefan Boltzmann Law
Electromagnetic radiation spreads out as it moves away from its source. As a result, the intensity of the radiation decreases as distance from the source becomes greater (as illustrated in Figure 5). The simplest example involves a point source of radiation (distance from the source is much greater than the size (e.g. surface area) of the emitter). With a point source, reduction in radiation intensity follows the inverse square law.
Figure 5. Radiation Intensity Decreases With Distance
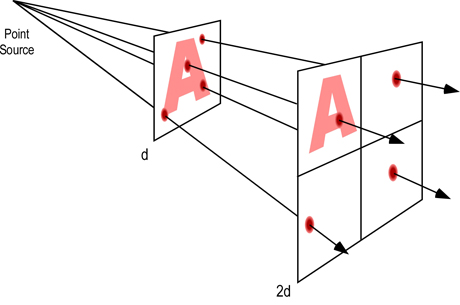
Inverse Square Law: For point sources, intensity of the radiation varies inversely with the square of the distance from the source. Doubling the distance reduces intensity of the radiation by a factor of four (1/4 of its original value).
When radiation is emitted from other than a point source (as it is under fire conditions), variation of the radiation intensity with distance is more complex. If the area of the source is large compared with the distances involved, intensity decreases with distance but does not follow a simple law. As a rough guide, if the distance from the source is greater than about 5 times the dimensions of the source, the inverse square law can be applied.
More to Follow
Subsequent posts in this series will examine physical and chemical changes and the process of combustion.
Ed Hartin, MS, EFO, MIFireE, CFO


