Everyday Concepts:
Energy, Heat, & Temperature-Part 2
I am using this series of posts to work through the process of developing a chapter on the foundational scientific concepts related to practical fire dynamics and fire control theory. My hope is to take the middle ground between the oversimplified and unsupported explanations provided in most texts intended for firefighter training and the higher level materials intended for fire protection engineers. This is proving to be no small task! Your feedback on my success (or lack thereof) in providing scientifically sound, but reasonably simple explanations would be greatly appreciated.
Back to Everyday Concepts Part 1
When faced with the challenge of developing firefighters understanding of energy, temperature, heat, and power in a limited timeframe, I generally avoid detailed discussion of the actual definition of the SI unit for energy, the Joule, and the mechanical equivalent of thermal energy. I have found that illustrating the concept of the Joule as it relates to thermal energy in terms of heating water to serve the purpose. However, as I looked back at the first post in this series, I think it would be useful to go back to the source, and examine James Joule’s experiments that made the connection to the equivalence of mechanical and thermal energy.
While not commonly used in scientific work, the American fire service has typically used the British thermal unit (Btu) as a measure of thermal energy. The Btu is defined in terms of the heating effect of energy transferred to water. One Btu is the energy required to raise the temperature of one pound of water by one degree Fahrenheit.
As discussed in the first post in this series, the SI unit of measure for energy is the Joule (J) which is defined in mechanical terms, but is applicable to all forms of energy.
In the mid 1800’s English physicist James Joule demonstrated the equivalence of mechanical and thermal energy by using a mechanical apparatus to stir water in an insulated container with paddles driven by a falling weight (see Figure 1).
Joule (1845) reported that based on analysis of data from a number of experiments, that expenditure of mechanical energy of 817 ft/lbs (the energy required to raise 817 pounds to a height of one foot) was the equivalent of an increase in temperature of one pound of water by one degree Fahrenheit. Conversion to SI units of measure is a bit complex, but 817 ft/lbs is equal to 1107 Newton/meters (the energy required to raise a mass of 1107 N to a height of 1 meter). While a non-standard measure of energy, the Newton/meter (N/m) provides a direct comparison to ft/lbs. In mechanical terms, a N/m equals the SI unit for energy, the Joule. Expressed in SI units, 1107 Joule of energy were required to raise the temperature of 0.454 kg (1.0 lbs) of water 0.56o C (1o F). This is quite close to the currently accepted conversion value in which 1055 J = 1 Btu.
Figure 1. Demonstration of the Mechanical Equivalent of Heat
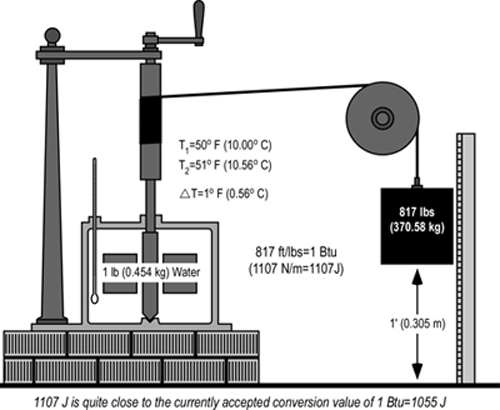
Note: Joule used a lesser weight falling over a greater distance, repeated a number of times. This drawing is simplified to provide a conceptual illustration.
Heat Transfer
In everyday language the word heat is used in a variety of ways (many of which are incorrect from a thermodynamic perspective). In thermodynamics, heat is a method of energy transfer. Heat is not a form of energy (a commonly stated misconception), but simply the name of the process of energy transfer based on temperature difference. Objects do not “have” heat, they have thermal energy, and heat is thermal energy in the process of transfer to objects having a lower temperature.
Even though it involves energy transfer, heat is not the same as work. Remember that work involves force causing movement in a direction influenced by that force (and if no movement in that direction occurred, no work is done). Energy transferred by heat results in an increase in molecular movement, but not in a specific direction, therefore no work is done. However, this does not mean that energy transferred by heat cannot be transformed into mechanical energy and accomplish work.
Transfer of energy from one object to another must be classified as heat or work. When energy content changes, it must be the result of heat, work, or a combination of both. Heat and work are processes by which energy is exchanged rather than energy itself.
The word flow is often used in discussing heat transfer (e.g., energy flows from objects with higher temperature to those with lower temperature). This helps visualize patterns of movement, but it is important to remember that neither energy nor heat is a fluid. Heat is the process of energy transfer due to temperature differences. This energy transfer takes place in a variety of different ways.
Second Law of Thermodynamics: There are several ways to state this law. The simplest is that heat cannot spontaneously flow from a material at lower temperature to a material at higher temperature. However, thermal energy moves from materials at high temperature to those having lower temperatures until they have the same temperature (equilibrium).
There are three methods of heat transfer, conduction, convection, and radiation. Each of these has significant impact on the processes of combustion, fire development, and fire control.
Conduction
Conduction of heat occurs when adjacent atoms vibrate against one another or as electrons move from atom to atom. Heat transfers through solid materials and between solid materials in direct contact with one another by conduction. The atoms in liquids and gases are further apart, reducing the probability of collision and transfer of thermal energy.
Figure 2. Conduction

Factors Influencing Conductive Heat Transfer
The factors influencing conduction are temperature difference, length (or thickness), cross sectional area, and the thermal conductivity of the conductor.
Thermal conductivity is the measure of the quantity of thermal energy which flows through a conductor. In addition to form, there are a number of factors influencing thermal conductivity of materials including molecular bonding, structure, and density. Units of measure for conductivity must account for the amount of energy transferred in a given amount of time, thickness (or distance), and temperature difference. The SI units of measure for thermal conductivity are Watts per Kelvin per Meter (W?K?m). While appearing to be complex, this measure is fairly straightforward; indicating the number of Watts (Joules/second) transferred a distance of one meter for each Kelvin of temperature difference (Figure 3)
Figure 3. Thermal Conductivity
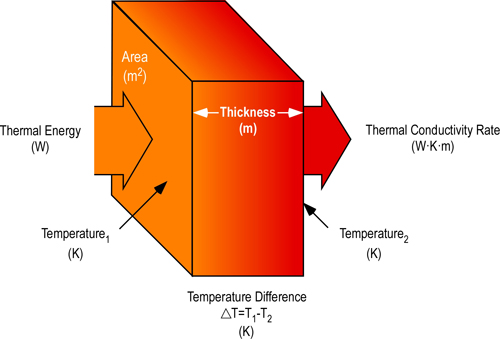
When the temperature of one surface of a solid material is higher than another, heat will move through the material. Depending on the characteristics of the material, this conductive heat transfer may be slow or it may occur quickly. The rate of heat transfer is defined by the coefficient of thermal conductivity.
As illustrated in Figure 3, the total amount of heat transfer is dependent on the coefficient of thermal conductivity, difference in temperature, and cross sectional area of the conductor. It is difficult to measure thermal conductivity as it describes a semi-static situation with a constant temperature gradient. However, heat transfer results in temperature changes towards equilibrium (equal temperature at all points in the conductor).
A high coefficient means heat moves very quickly; a low coefficient means heat moves very slowly. As illustrated in Table 1, the thermal conductivity constant (k) for different materials varies considerably.
Table 1Thermal Conductivity Table
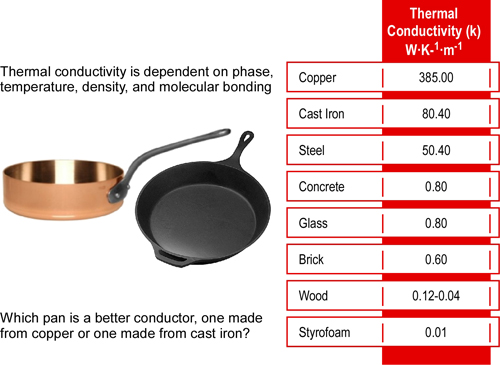
Metals are usually the best conductors of thermal energy due to their molecular bonding and structure. Metallic chemical bonds have free-moving electrons and form a crystalline structure which aids in transfer of thermal energy as illustrated in Figure 4.
Figure 4. Conduction in Metals
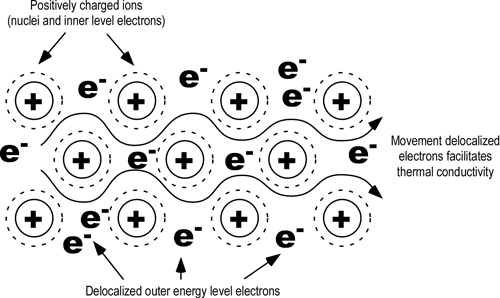
Because the outer electrons in metals are shared by all the atoms, they are not considered to be associated with any one atom. Since these electrons are attracted to many atoms, they have considerable mobility that allows for the good thermal conductivity seen in metals.
In general, density decreases so does conduction (some unusual materials such as carbon foam, have low density and high conductivity). Therefore, most fluids (and especially gases) are less conductive. This is due to the large distance between atoms in a gas: fewer collisions between atoms means less conduction. Conduction is dependent on the area being heated, temperature differential, and thermal conductivity of the material.
What’s Next
The next post in this series will examine convection and radiation as mechanisms of heat transfer. In addition, I will be starting a series of posts to discuss a comprehensive approach for nozzle testing from an operational perspective.
Ed Hartin, MS, EFO, MIFireE, CFO
References
Joule, J. (1845). On the existence of an equivalent relation between heat and the ordinary forms of mechanical power. Philosophical Magazine, 3(xxvii), p. 205.
Tags: fire behavior, Fire Behavior Training, practical fire dynamics



April 14th, 2010 at 02:28
Hello Ed
You wrote: “In thermodynamics, heat Is a method of energy transfer. Heat is not a form of energy (a misconception Commonly Stated ),….”
I do not agree with that …
In my point of view, the heat, is not equated to a method of energy transfer but to a quantity of energy transferred. The methods of energy transfer are : conduction, convection and radiation.
Fr@+nck
April 14th, 2010 at 05:27
Franck, My choice of the word “method” could have been better. My point was that heat is energy transfer due to temperature difference (e.g., as differentiated from mechanical means). It would likely have been more appropriate to say that heat relates to energy transfer. Thanks for pointing this out. Cheers, Ed
April 14th, 2010 at 05:38
OK Ed, thank you for your reply. best.
Greetings
Fr@+nck