Everyday Concepts: Energy, Heat, & Temperature-Part 1
Everyday Concepts
Firefighters, like most everyone else, have a commonsense, everyday understanding of energy, heat and temperature. However, this everyday understanding is likely to be considerably different than the way these concepts are defined and used in science. Think about how heat and temperature are used on a day-to-day basis. On a sunny summer day, people are likely to say that it is hot because they feel hot or because the thermometer indicates a temperature is high. This may lead people to believe that temperature is a measure of hotness or heat. On the other hand, scientists view these concepts considerably differently!
So what! What difference does it make if we use our commonsense, everyday understanding of energy, heat, and temperature in our effort to make sense of fire dynamics? Why is this important?
If you are going to take a trip, it is useful to understand the concept of distance and have some type of units (e.g., kilometers or miles) to describe how far away your destination is. When describing a building, firefighters indicate the number of stories and dimensions (e.g., meters or feet). Having a good grasp of the concepts of energy, heat and temperature provides a way to describe the fire potential of different types of fuel, the size of a fire in terms of energy and power, and the thermal environment encountered by firefighters.
Take a minute and think about how you would define energy, heat, and temperature. Write your ideas down on a piece of paper so you can come back to them later. Don’t worry about the textbook definition, just write down what you think these words mean. After reading the rest of this post, come back to your notes and see how your understanding of energy, heat, and temperature has changed.
Energy, Heat, and Temperature in Firefighting
For many years, firefighters in the United States learned about British thermal units (Btu) as a measure of energy. A British thermal unit is the amount of energy required to raise the temperature of 1 pound of water (at 60o Fahrenheit (F)) 1o F. Firefighters often can state a reasonable approximation of this definition and the Btu seems to be a fairly simple unit of measure with direct applicability in the firefighting context.
“Before an observer can formulate and assent to an observation statement, he or she must be in possession of the appropriate conceptual framework and a knowledge of how to appropriately apply it” (Chalmers, 1999, p. 11). It is one thing to recognize a definition, but it is another thing entirely to be able to use this information in a broader context and make sense of things! For example a young child may be able to identify a red apple, but may not have a good understanding of what makes this fruit an apple (as opposed to a pear) or how a red apple and a green apple can both be apples. Developing an understanding of the fundamental scientific concepts that underlie fire dynamics and firefighting is much the same. Knowledge and understanding must extend beyond simple recognition of, or the ability to restate definitions and concepts presented in a text of lecture.
Thermodynamics
Thermodynamics is a branch of physics that describes processes that involve changes in temperature, transformation of energy, and the relationships between heat and work. Fire and firefighting also involves changes in temperature, transformation of energy, heat and work. “Thermodynamics, like much of the rest of science, takes terms with an everyday meaning and sharpens them – some would say, hijacks them – so that they take on an exact unambiguous meaning” (Atkins, 2007, p. 3).
Thermodynamics deals with systems. A thermodynamic system is one that interacts and exchanges energy with the area around it. A system could be as simple as a block of metal or as complex as a compartment fire. Outside the system are its surroundings. The system and its surroundings comprise the universe.
While in general terms the universe includes everything, we will generally look at things on a smaller scale. For example we might consider a burning fuel package as the system and the compartment as the surroundings. On a larger scale we might consider the building containing the fire as the system and the exterior environment as the surroundings.
Figure 1. Thermodynamic Systems
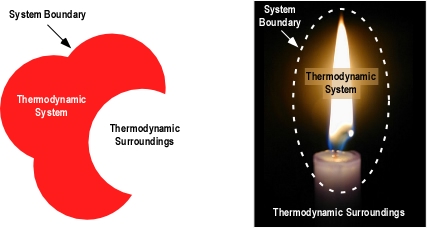
Thermodynamic systems can be classified on the basis of their interaction with the surroundings.
- Isolated systems do not exchange energy or matter with their environment.
- Closed systems exchange energy but not matter with their environment.
- Open systems exchange energy and matter with their environment. A boundary allowing matter exchange is called permeable.
Laws of Thermodynamics: These laws summarize the properties of energy and its transformation from one form to another. Numbered from zero to three, these laws are both simple and extremely complex. This series of posts examines the laws of thermodynamics in the context of fires and firefighting to move from theoretical to practical application.
Energy
Energy is a fundamental concept in physical science, but is difficult to define in a way that is meaningful on an everyday basis. Energy is the ability to do mechanical work or transfer thermal energy from one object to another. Energy can only be measured on the basis of its effects. There are basically two kinds of energy, kinetic and potential. Kinetic energy is associated with motion of an object and potential energy is that which is stored and may be released at a later time.
There are a number of different forms of energy; mechanical, chemical, electrical, radiant, and thermal. However, each has the ability to be transformed into work, which is force applied to an object, causing it to be displaced. In thinking about energy and work it is important to keep two things in mind:
- Energy is the capacity to do work.
- Work involves force causing movement in the direction of that force.
If the force does not influence movement in the direction of the force, no work was done.
Newtons (named after Isaac Newton) are the standard international (SI) unit for force. A Newton is the amount of force required to give a mass of one kilogram an acceleration of one meter per second squared. However, it may be easier to visualize force in terms of weight. In our everyday environment, weight is the force exerted as a result of our mass and the effects of gravity. For example, a kilogram (which is a unit of mass) exerts a downward force of 10 Newtons (or 2.2 pounds). To make things more complicated, kilograms are used in everyday language to express weight (rather than Newtons). This is because on earth, weight and mass are directly proportional.
The SI unit for energy (capacity to do work) is the Joule. A Joule is a force of one Newton causing displacement of an object a distance of one meter. For example, approximately one Joule of energy is required to lift a small apple (which weighs one Newton (or 0.22 pounds) a distance of 1 meter. In that energy is the capacity to do work, the Joule is also used to measure energy (regardless of its form).
While mechanical energy may be of interest to firefighters, what does this have to do with thermal energy and fire behavior? One really big puzzle is how Joules which are defined in terms of mechanical energy can be used to measure thermal energy? This is a really good question, but several more scientific concepts are needed in order to make sense of the answer.
Substances have potential chemical energy based on the bonds within and between their atoms and molecules. Formation, breaking, or rearrangement of these chemical bonds results in transfer of energy into or out of the substance. For example, in combustion the reaction of an oxidizer and fuel results in transformation of chemical potential energy into thermal and radiant kinetic energy. Thermal energy is molecular kinetic energy resulting from molecules moving around in random directions as well as molecular rotation and vibration. Radiant energy is comprised of electromagnetic waves in the infrared region of the electromagnetic spectrum although some is in the visible region. The term thermal radiation distinguishes this form of electromagnetic radiation from other forms such as radio waves and ionizing radiation
First Law of Thermodynamics: Energy cannot be created nor destroyed only transformed from one form to another. For example, in combustion the chemical reaction between oxygen and fuel results in transformation of chemical energy to thermal and radiant energy. However, the total amount of energy remains the same.
Temperature
Temperature is a measure of the average kinetic energy. Temperature of any substance, whether solid, liquid, or gas, is directly related to all motion (kinetic energy) of its molecules. This is especially important for liquids and solids because the kinetic energy of these substances is almost entirely vibrational and rotational. All molecules above a temperature of absolute zero (the temperature at which molecular motion stops) are in a continual state of motion and possess kinetic energy.
The Kelvin is the standard international unit for temperature. In this scale, temperature is measured in Kelvins (K), not degrees (as with the Celsius and Fahrenheit scales). While the least common in everyday use, the Kelvin thermodynamic temperature scale is important in understanding thermal energy, temperature, and heat. With the Kelvin scale, 0 K is absolute zero, the theoretical absence of all thermal energy.
While the Kelvin is the standard international unit for temperature, the Celsius scale is commonly used as both an everyday (outside the United States) and scientific measure of temperature. The degree Celsius (o C) is the same increment of measure as the Kelvin, the difference between these two scales is the zero point on the scale. With the Celsius scale 0o C is the freezing point of water (273.15 K) while as previously noted 0 K (-273.16o C) is absolute zero.
In the United States, the Fahrenheit scale is commonly used to measure temperature on an everyday basis. Unlike the Celsius scale where the difference between the freezing and boiling points of water is 100o, the Fahrenheit scale places the freezing point at 32o and boiling point at 212o, a difference of 180o.
Figure 2. Common Temperature Scales
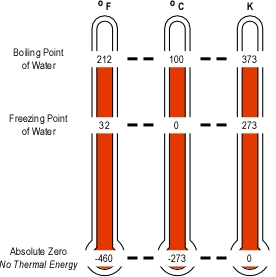
Note: Equivalent temperatures have been rounded to the closest whole unit (i.e., degree, kelvin).
The Kelvin temperature scale is used in scientific work involving thermodynamics, because this scale starts at absolute zero (the point at which a substance has no thermal energy). This means that temperature in Kelvins is a measure of the absolute temperature. Use of an absolute temperature scale allows expression of physical laws and mathematical formulas more simply.
For example, 100o C is not twice as high a temperature as 50o C (even though at first glance it appears that it is). This becomes clear when using the Kelvin scale. A temperature of 50o C is 323.15 K while 100o C is 373.15 K, an increase of just over 13% in absolute temperature.
Third Law of Thermodynamics: In the complete absence of molecular kinetic energy, the temperature of a substance would be absolute zero. Absolute is 0 K or -273.15° C.
Measuring Energy
As previously discussed, the SI unit of measure for energy is the Joule (J). While defined in terms of mechanical work, the joule is used for all forms of energy. In the standard international system of units, prefixes such as kilo (thousand) and mega (million) are used to provide incrementally larger units of measure. In the case of energy, a kilojoule (kJ) would be a thousand joules and a megajoule (MJ) would be one million joules.
While not commonly used in scientific work, the American fire service has typically used the British thermal unit (Btu) as a measure of thermal energy. The Btu is defined in terms of the heating effect of energy transferred to water. In order to provide a simple explanation of the Joule as a unit of measure for thermal energy and allow a direct comparison to the Btu, Figure 3 describes the J in terms of energy transfer to water and provides a comparison to the Btu.
Figure 3. Joule and British Thermal Unit
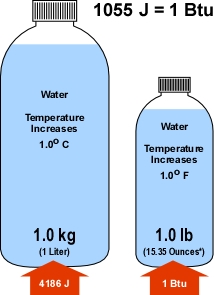
* In this case, Ounces is a measure of volume not mass (or weight). Another confusing aspect of the traditional system of measure used in the United States!
As illustrated in Figure 3, addition of 4186 joule of energy to a kilogram of water raises its temperature (average internal kinetic energy) by one degree Celsius. Similarly, adding one British thermal unit of energy to a pound of water raises its temperature by one degree Fahrenheit. Directly comparing these two examples is a bit complex as the units of measure for both energy (J & Btu) and temperature (o C & o F) are different.
Some properties of materials are independent of their mass, color would be one example. Other properties are dependent on mass. Weight, would be the most obvious, but other properties are also dependent on the mass of material present.
Figure 4. Energy and Temperature Simulation
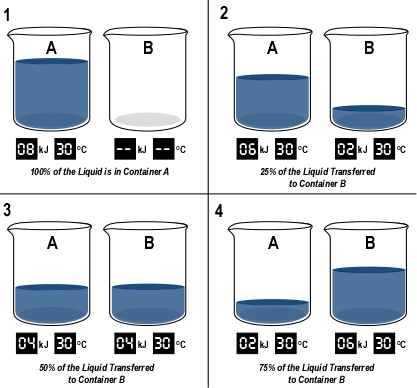
Note: This illustration was adapted from a simulation in Energy: Thermal Energy, Heat and Temperature, a National Science Teachers Association knowledge object.
The example provided in Figure 4 examines the difference between temperature and thermal energy as related to mass. The container labeled A initially contains a specific mass of liquid with a temperature of 30o C and a total thermal energy of 8 J. Liquid is moved from the container labeled A to the one labeled B. How does the temperature of the liquid and thermal energy in each container change as this transfer takes place? The temperature of the liquid remains the same regardless of the quantity in each of the containers. However, as the mass of liquid in each container changes, the thermal energy of the liquid in the container changes as well. Related to average thermal energy, temperature is independent of mass, while the total thermal energy relates directly to mass. Properties of materials fall into two categories. Extensive properties (like energy) are dependent on the quantity (mass) of material while intensive properties (like temperature) are not.
What’s Next?
The next post in this series will examine the concept of heat and the relationship between heat, energy, and temperature.
Ed Hartin, MS, EFO, MIFireE, CFO
References
Atkins, P. (2000). Four laws that drive the universe. Oxford, UK: Oxford University Press
Chalmers, A. (1999) What is this thing called science? (3rd ed.). Indianapolis, IN: Hackett.
National Science Teachers Association (NSTA). (2006). Energy: Thermal energy, heat and temperature. Retrieved February 27, 2010 from http://www.nsta.org/store/product_detail.aspx?id=10.2505/7/SCB-EN.3.1
Tags: fire behavior, Fire Behavior Training, practical fire dynamics



February 28th, 2010 at 09:37
Chief Hartin, I’m confused about your figure 3 example. Is the Joule under the 1 kg (liter) supposed to read 1 Joule or is 4186 Joule correct?Brian
February 28th, 2010 at 13:26
Brian, It taks 4186 J to raise the temperature of 1 kg (liter) of water 1degree C. Comparing this description to the Btu is somewhat complicated by the fact that the units for mass, temperature, and energy are all different. Ed
March 21st, 2010 at 18:10
[…] to thermal energy in terms of heating water to serve the purpose. However, as I looked back at the first post in this series, I think it would be useful to go back to the source, and examine James Joule’s experiments that […]