Gas Explosions–Part 2
My last post (Gas Explosions) examined flammability and ignition of fuel/air mixtures as related to gas explosions. Deflagration of a fuel/air mixture can result in a significant energy release, when confined, this results in a significant pressure increase.
Pressure
If a confined gas is heated, pressure will increase as indicated in Gay-Lussac’s Law.
Gay-Lussac’s Law: When the volume of a gas remains the same and temperature is increases, pressure increases in proportion to the absolute temperature of the gas.
Pressure generated in a gas explosion is dependent on the speed with which flames move through the fuel and the degree to which expanding hot gases are confined.
The speed with which flames propagate through unburned pyrolysis and flammable combustion products is subsonic (slower than the speed of sound), making this a deflagration. Flame propagation in backdraft may be several meters per second (Guigay, G., Eliasson, J., Gojkovic, D., Bengtsson,L., & Karlsson, B., 2008). The pressure generated by this type of explosion inside a compartment or building can easily break windows (changing the ventilation profile) and in many cases can be sufficient to result in structural damage.
When pre-mixed fuel and air is ignited, it pushes unburned bas ahead of the flame, producing turbulence. Flame propagation into this turbulent, pre-mixed fuel will result in an increased rate of combustion, increasing velocity and turbulence even further. This feedback loop results in acceleration of flaming combustion and high pressure from expansion of hot gases. When this reaction is confined (e.g., ventilation is limited to a single opening such as a door or window), pressure can increase to an even greater extent.
In an explosion of unburned pyrolysis and combustion products and air, the severity of the reaction will depend on the total mass and concentration of fuel, location of the ignition point, strength of the ignition source, and extent of confinement. While it is not possible to evaluate these factors under fire conditions, understanding the variables aids in understanding the processes involved. For example, as illustrated in Figures 1 and 2, ignition that occurs inside a compartment can expel a mass of unburned fuel which may subsequently ignite (note that the opening may not be to the exterior, but may simply be to another interior compartment, stairwell, etc.).
Figure 1. Influence of Ignition Location

Expulsion of unburned gas phase fuel from a compartment in a backdraft results in a characteristic spherical mass of fuel (as illustrated in Figure 2) which subsequently ignites, resulting in a fireball.
Figure 2. Expulsion of Gas Phase Fuel in a Backdraft

How might ignition location have influenced the nature and duration of flaming combustion in the stairwell in the Watts Street incident discussed in 15 Years Ago: Backdraft at 62 Watts Street and 62 Watts Street: Modeling the Backdraft?
Thermal and Structural Effects of Explosions
Explosions and the resulting pressure increase occur extremely rapidly, this makes the force that is applied to structures a dynamic load. How a structure responds to this type of dynamic load depends on the magnitude of the load, design, and condition of the structure before the load was applied. In addition to the pressure generated by an explosion, movement of gas at high velocity also adds to the dynamic load imposed on the structure. Figure 4 illustrates the rapid changes in pressure resulting from an explosion in a compartment.
Figure 4. Explosion Time-Pressure Curve
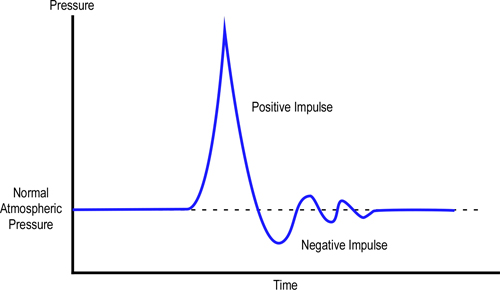
Note: Adapted from the Gas Explosion Handbook (GexCon, 2006).
Backdraft and smoke explosion can generate considerably more pressure and flow than is necessary to cause structural damage. Even if the pressure from an explosion is limited, it will generally be sufficient to cause failure of window glazing or damage to other building openings, resulting in a significant change in ventilation profile. When the fire is ventilation controlled, this will lead to increased heat release rate and potential for rapid transition to a fully developed fire.
Ed Hartin, MS, EFO, MIFireE, CFO
References
GexCon. (2006) Gas explosion handbook. Retreived March 20, 2009 from http://www.gexcon.com/index.php?src=handbook/GEXHBchap4.htm
Guigay, G., Eliasson, J., Gojkovic, D., Bengtsson,L., & Karlsson, B. (2008) The Use of CFD Calculations to Evaluate Fire-Fighting Tactics in a Possible Backdraft Situation. Fire Technology
Tags: backdraft, Extreme Fire Behavior, fire behavior, smoke explosion



July 25th, 2009 at 07:42
[…] For additional information on transient, explosive, fire phenomena see earlier posts: Gas Explosions and Gas Explosions Part 2. […]