Gas Explosions
Extreme fire behavior can be categorized as a step event which results in a sustained increase in heat release rate or a transient event that results in a brief increase in heat release rate. Transient events involve combustion of unburned combustion and pyrolysis products. The speed of this combustion process can vary widely depending on the concentration of fuel and oxygen, extent of mixing, confinement and a number of other factors. Transient events may simply involve rapid combustion (e.g., flash fire) or they may be explosive (e.g., backdraft, smoke explosion), resulting in a significant pressure increase within the compartment or building.
My next few posts will provide brief overview of gas explosions in general to provide a foundation for understanding explosive extreme fire behavior phenomena such as backdraft and smoke explosion.
Introduction
The Gas Explosion Handbook (GexCon, 2006) defines a gas explosion as a process where combustion of premixed gas phase fuel and an oxidizer (e.g., fuel and air) causes a rapid increase in pressure. The fuel in a gas explosion may result from release of a flammable gas normally used as a fuel or in industrial processes (e.g., methane, cyclohexane) or from accumulation of unburned pyrolysis and combustion products in a compartment fire.
An explosion involving unburned pyrolysis and combustion products in a compartment fire may occur in one of two ways: 1) air is mixed with a rich fuel/air mixture and subsequently undergoes auto or piloted ignition (backdraft), or 2) a pre-mixed, flammable, fuel/air mixture undergoes piloted ignition (smoke explosion). Exploring the basic processes involved in a gas explosion will lay a foundation for understanding these two important extreme fire behavior phenomena.
Flammable Fuel/Air Mixtures in Compartment Fires
Compartment fires generally involve combustion of natural and synthetic organic (carbon containing) materials such as wood, paper, and plastics. In order for flaming combustion to occur, fuel must be transformed into the gas phase through vaporization or pyrolysis. Incomplete combustion of organic fuels results in production of carbon monoxide, soot, and a wide range of other products of combustion (many of which are flammable). Smoke is comprised of not only the products of incomplete combustion, but also unburned pyrolysis products. As illustrated in Figure 1, Smoke is Fuel!
Figure 1. Smoke is Fuel

Gas phase fuel in smoke may ignite and burn in the plume or ceiling jet or it may burn as it exits through a ventilation opening. However, unburned gas phase fuel may also accumulate inside the compartment or building, mixing with air to form a potentially flammable mixture. In ventilation controlled fires, concentration of gas phase fuel increases and may become too rich to burn without introduction of additional air. In addition, flammable products of combustion and pyrolysis products may infiltrate into uninvolved compartments or attached exposures and mix with air to form a flammable atmosphere.
Review of Flammability
Combustion requires fuel and oxygen in the proper concentration. Under normal conditions, air contains approximately 21% oxygen and 79% nitrogen and trace amounts of other gases. The nitrogen, other gases, and water vapor are passive agents as they are not chemically part of the combustion reaction (but as energy is required to raise the temperature of passive agents, they do influence combustion).
Figure 1. Methane Flammability Diagram
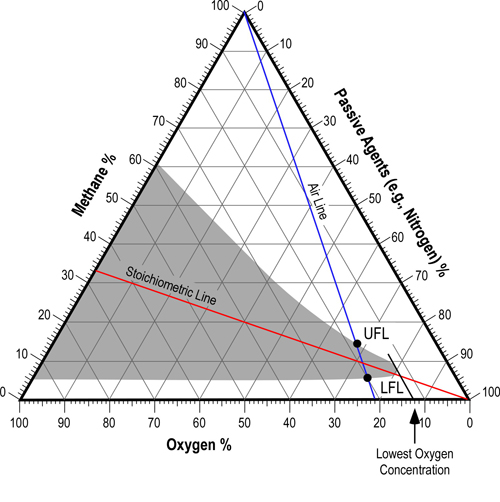
At first glance, the flammability diagram in Figure 2 appears to be extremely complex. However, it simply represents the relationship between fuel, oxygen, and passive agents. In this triangular diagram, the total of the concentration of fuel, oxygen, and passive agents equals 100%. In the case of Figure 2, the triangular diagram shows all possible mixtures of methane, oxygen, and nitrogen passive agents (predominantly nitrogen in the air). The blue (air) line indicates oxygen concentration from normal 21% (by volume) to 0%. The red (stoichiometric) line indicates the ideal mixture of oxygen and fuel for complete combustion. The gray shaded area indicates the mixtures of methane, oxygen, and passive agents that will be flammable.
The area of this diagram that is of greatest interest in most compartment fires is the region to the right of the Air Line (flammable limits under normal conditions and the minimum oxygen concentration that will allow combustion). This is because most compartment fires are dependent on ambient air as a source of oxygen. If the concentration of fuel increases, it must be offset by a corresponding reduction in oxygen and passive agents and may make the mixture too rich to burn. However, if fuel escapes and is replaced with air, the concentration of the mixing gases may reenter the flammable range.
The flammability diagram applies to pre-mixed fuel and air (oxygen and passive agents). In a compartment fire, the concentration and mixing of fuel and air varies considerably due to differences in temperature and resulting density of smoke and air. Consequently, there may be pockets of fuel and air that are within the flammable envelope, while other areas may be too rich or lean to burn.
Ignition of Fuel/Air Mixtures
If smoke is flammable, why doesn’t it always ignite and burn? Ignition is dependent on having sufficient fuel and oxygen as well as an adequate ignition source. Ignition of a mixture of pre-mixed air and fuel requires an ignition source with sufficient strength. The minimum amount of energy required to initiate combustion is the minimum ignition energy. Factors that affect the minimum ignition energy include:
- Type of fuel
- Mixture of fuel and air
- Temperature
- Total energy supplied
- Rate at which energy is supplied (energy per unit time)
- Area over which energy is delivered
The minimum ignition energy for a given fuel generally corresponds to the stoichiometric (ideal) mixture of fuel and air. As concentration increases or decreases within the flammable range, ignition energy increases (i.e., ignition energy at the Lower and Upper Flammable Limits will be higher than for the stoichiometric concentration).
The concentration and specific gas species of flammable combustion and pyrolysis products is complex and will influence the energy required for ignition. The concept of ignition energy and the influence of concentration of fuel in air is important in understanding why a flammable mixture of combustion and pyrolysis products may not be ignited by surface combustion, but may be ignited by the higher energy provided by flames.
More to Follow
My next post will continue with a look at other factors that influence explosive ombustion in compartment fires.
Ed Hartin, MS, EFO, MIFireE, CFO
References
GexCon. (2006) Gas explosion handbook. Retreived March 20, 2009 from http://www.gexcon.com/index.php?src=handbook/GEXHBchap4.htm
Tags: backdraft, Extreme Fire Behavior, fire behavior, smoke explosion



June 22nd, 2009 at 22:12
[…] additional information on transient, explosive, fire phenomena see earlier posts: Gas Explosions and Gas Explosions Part […]
September 9th, 2009 at 06:35
[…] Gas Explosions […]
November 26th, 2009 at 06:29
I am currently part of the real fire training dept within the London Fire Brigade and have received the following enquiry, ‘what is a passive agent and where does the term come from?’
We currently teach that the walls, furniture etc within the fire compartment are passive agents, absorbing the heat and not contributing directly to the combustion process. Once they stop absorbing heat and begin to radiate heat back in to the fire compartment they are no longer passive but active agents.
The question posed was ‘Are the items of furniture etc passive agents or are they unburnt fuel? and where does the term ‘passive agent’ come from?’
November 26th, 2009 at 11:58
John, Passive agents or thermal ballast (synonym) are materials in the fire environment that are not participating in the combustion reaction (usually because of their chemical makeup). In air, the mass of the gases other than oxygen acts as a passive agent (which must be heated, but does not participate in the oxidation reaction). Water introduced for cooling is also a passive agent (must be heated, but does not participate in combustion). I would not consider fuel to be a passive agent in the strictest sense of the concept, but energy is transferred to fuel packages prior to sufficient pyrolysis. One way to see this effect is to place small coil of copper wire in a candle flame, the wire can absorb sufficient energy to result in extinguishment (I generally use wire about 2 mm in diameter formed into a 6 to 8 mm coil for this demonstration). Another example (which would likely be less useful in urban environments is the impact of fuel moisture (water being a passive agent/thermal ballast). It is much more difficult to ignite wood with a high moisture content as the water must first be driven from the fuel (slowing temperature increase to the ignition temperature). I first encountered the terms passive agent and thermal ballast while attending fire behavior instructor training in Uppsala, Sweden. I will dig a bit more into the origin of the term. Hope that this helps! Cheers, Ed
March 6th, 2010 at 09:39
[…] Gas Explosions […]