FAQ-Fire Attack Questions
Sunday, April 14th, 2013Captain Mike Sullivan with the Mississauga Ontario Fire Department and I are continuing our dialog with another series of questions related to the science behind fire attack and fire control methods.
The first several questions pertain to the video produced by the Kill the Flashover project illustrating the impact of anti-ventilation on heat release rate and compartment temperature.
Would you happen to know what type of building this was done in (house or concrete burn building) and what fuel was used?
KTF 2011 and 2012 were conducted in acquired structures and KTF 2013 was conducted in a purpose built burn building. Each of the KTF burns used normal types of building contents to provide realistic fire conditions for the demonstrations/experiments. The first burn in KTF 2011 use a fuel load consisting of a chair, small amount of wood, carpet and carpet pad (as illustrated below).

You mentioned in the “Kill the Flashover” video about the key to heat reduction is the lack of oxygen for the heat release. I understand this but still wonder where all this heat goes, does it not have to dissipate somewhere?
The following video was shot during the first burn in KTF 2011. As previously discussed, the fuel load was comprised of a chair, carpet, carpet pad, and a small amount of wood. At the start of the burn the only opening to the compartment was a typical sized residential doorway. After the fire became well developed the door was closed.
You are absolutely correct! A compartment fire is an open thermodynamic system in which there is an ongoing transfer of mass (e.g., smoke out and air in) and energy between the system and its environment. This leads to another excellent question.
We often speak about fire and how the box “can’t absorb any more heat” and this is usually the point where we start to near flashover, this is what we thought was occurring, the box was simply continuing to absorb heat.
The phrase “can’t absorb any more heat” is scientifically incorrect, unless the “box” and the flames or hot gases are all of equal temperature. If any portion of the compartment or fuel packages within the compartment are lower than the temperature of flames or hot gases, the temperature of this matter will continue to increase (until thermal equilibrium is reached). The oversimplified explanation likely relates to the endothermic (heat absorbing) process of pyrolysis and transition to the exothermic (heat releasing) process of combustion.
Any object with a temperature above absolute zero transfers thermal energy to objects having a lower temperature. In a compartment fire energy released by the combustion reaction is transferred to materials within the thermodynamic system through radiation, conduction, and convection (as illustrated below).
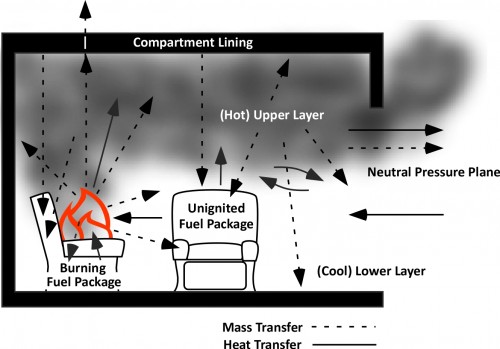
Under fire conditions, increasing temperature in the compartment is the result conversion of chemical potential energy in the fuel to thermal energy through combustion. When the rate of energy released exceeds losses of thermal energy to the thermodynamic surroundings, temperature increases. When heat release rate is reduced by limiting the oxygen available for combustion (i.e. closing the door), continued transfer of energy to the thermodynamic surroundings results in a drop in temperature.
This is somewhat like bringing a pot of water on the stove to a boil and then removing it from the burner. Once off the burner, the water continues to transfer energy to its surroundings and will begin to cool.

FAQ-Fire Attack Questions will continue next week with a discussion of gas cooling, fog patterns and solid or straight streams, and limitations encountered when working in large volume spaces!.


